The materials used in healthcare and cleanroom environments—from filtration media to protective garments—are literally the last line of defense against microbial contamination and infection. The reliability of these barriers is not merely a matter of quality; it is a direct determinant of patient and provider safety, making stringent, third-party medical textile testing one of the most vital processes in the manufacturing supply chain. If your facility produces high-performance filtration media, protective apparel, or nonwoven textiles for clinical use, a deep understanding of current regulatory requirements and advanced analytical techniques is necessary to guarantee product function and compliance. This long-form article outlines the core testing methodologies, regulatory frameworks, and specialized laboratory analyses required to certify the performance, sterility, and durability of products like surgical masks, respiratory filters, and hospital gowns.
Assessing Filtration Performance and Barrier Efficacy (VFE)
For products designed to block airborne particulates and biological contaminants—such as surgical masks, respirators, and air filtration media—the most essential testing category is filtration efficiency. This family of tests provides quantifiable data on the material’s ability to act as a physical barrier against microorganisms and aerosols. Failure in this area renders the product useless for its intended purpose and poses immediate risks in clinical settings.
The key measure of performance for respiratory protection devices and related media is Viral Filtration Efficiency (VFE) and Bacterial Filtration Efficiency (BFE).
Core Filtration Efficacy Tests
| Test Type | Target Material | Standard Protocol (Example) | Key Performance Measure |
|---|---|---|---|
| Bacterial Filtration Efficiency (BFE) | Surgical Masks, Gowns | ASTM F2101 | Measures percentage of Staphylococcus aureus (average size 3.0 μm) filtered by the material. |
| Viral Filtration Efficiency (VFE) | Respirators, Face Masks | ASTM F2101 Modified | Measures percentage of bacteriophage ΦX−174 (average size 0.027 μm) filtered. |
| Particle Filtration Efficiency (PFE) | Cleanroom Filters, Media | ASTM F2299 | Measures the efficiency of the material against non-viable particle sizes (e.g., 0.1 to 5.0 μm). |
| Differential Pressure (ΔP) | All Barrier Textiles | MIL-M-36954C | Measures air permeability (breathability), ensuring filtration does not compromise user comfort or safety. |
The viral filtration efficiency (VFE) test is particularly crucial because viruses are significantly smaller than bacteria, making them the ultimate challenge for fine filter media. A high VFE rating is non-negotiable for any product claiming protection against airborne viral pathogens. Furthermore, labs must ensure that the material’s air permeability (measured via ΔP) remains low enough for user safety while still providing excellent filtration, offering a critical balance between breathability and protection.
Material Safety, Biocompatibility, and Chemical Purity
When textiles are used in clinical settings—especially those designed for direct, prolonged skin contact like hospital gowns and wound dressings—ensuring material safety is paramount. These materials must be biocompatible, meaning they do not irritate the skin, cause allergic reactions, or release toxic substances upon contact with human tissue or fluids.
Rigorous biocompatibility testing is conducted following guidelines such as ISO 10993, which requires a series of in vitro and in vivo tests depending on the product’s contact duration and environment. Outsourcing these advanced tests is often the most efficient route for manufacturers to ensure compliance and patient safety.
Essential Material Safety Testing Components:
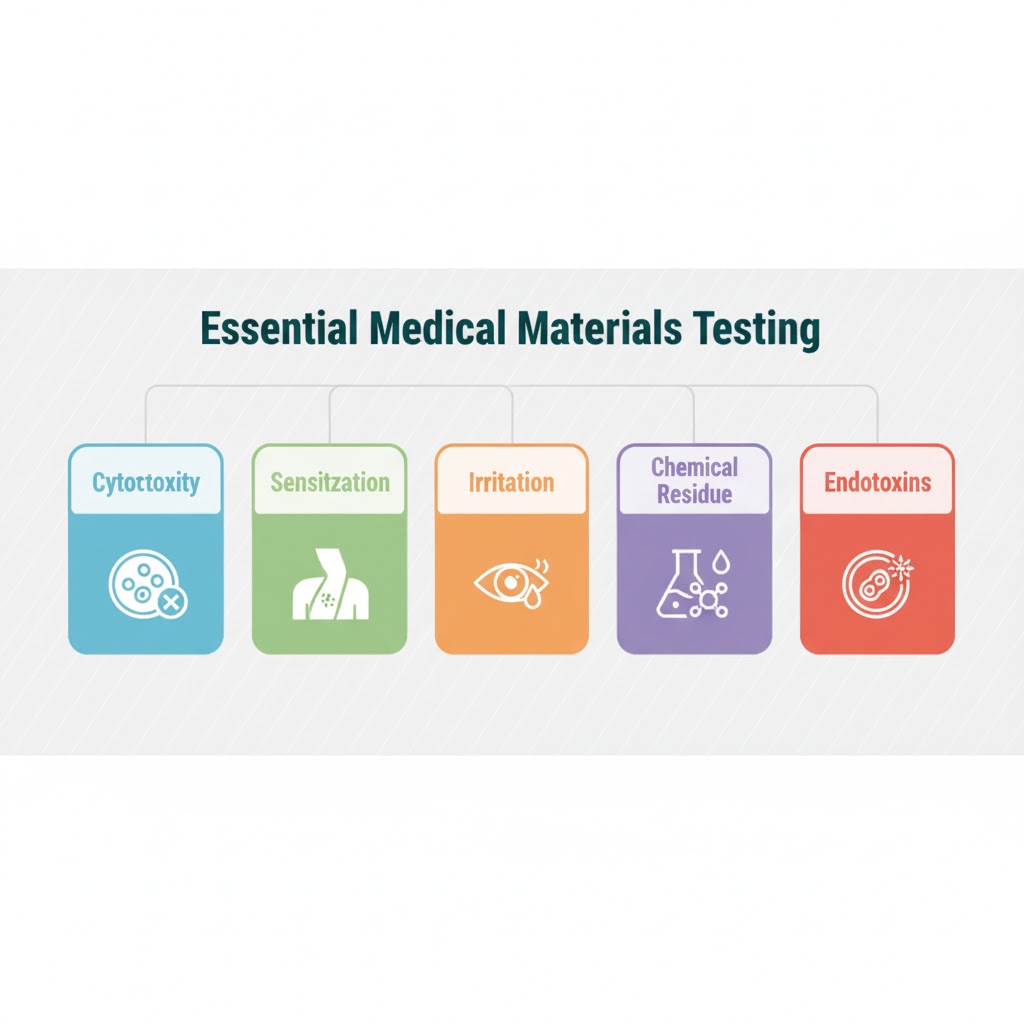
- Cytotoxicity Testing (ISO 10993-5): Evaluates whether the materials extract substances that are toxic to cells, a fundamental safety check for all medical devices.
- Sensitization (ISO 10993-10): Determines the potential for the textile or filter to cause skin allergies after repeated exposure.
- Irritation (ISO 10993-10): Assesses the material’s capacity to cause local irritation when in contact with the skin or mucous membranes.
- Chemical Residue Analysis: Screening for residual processing chemicals, heavy metals, or plasticizers that might leach out of the material. This is crucial for surgical masks and all high-contact products. Techniques like HPLC and GC-MS are used to detect trace amounts of restricted chemicals.
- Endotoxin Testing (LAL Assay): Measures the presence of bacterial endotoxins, which can cause severe inflammatory reactions if present on implantable materials or sterile products.
A comprehensive medical textile testing program must address all aspects of chemical and biological safety to guarantee patient well-being and meet stringent regulatory requirements across global markets, firmly establishing material safety as a core quality metric.
Validation of Sterilization Protocols and Microbial Challenge
For any medical textile testing concerning invasive procedures, the product must be delivered to the end-user in a certified sterile state. This is true for many hospital gowns, surgical drapes, and certain types of advanced wound dressings. Sterilization efficacy testing is therefore a non-negotiable pillar of compliance, validating the entire sterilization process (e.g., Ethylene Oxide, gamma irradiation, steam, or e-beam).
The core of sterilization validation lies in the use of Biological Indicators (BIs), which are highly resistant microbial spores used to challenge the sterilization cycle. If the BIs are killed, the process is deemed effective.
Key Sterilization Validation Steps:
- Bioburden Determination: Before sterilization, the product’s microbial load (bioburden) must be accurately quantified. This data is essential for determining the necessary radiation dose or cycle time.
- Procedure: Quantitative recovery of viable microorganisms from the product using validated extraction methods.
- Sterility Assurance Level (SAL) Verification: Validation aims to achieve a minimum SAL of 10−6, meaning there is less than a one in a million chance of a single non-sterile unit.
- Residual Analysis: For chemical methods like Ethylene Oxide (EtO), thorough testing for residual EtO is required. High levels of residue pose a direct material safety risk to patients and staff.
- Package Integrity Testing: Sterility is only maintained if the packaging remains intact. Burst strength, seal strength, and dye penetration tests ensure the package acts as a reliable microbial barrier until the point of use.
Outsourcing medical textile testing for sterilization protocols ensures adherence to standards like ISO 11135 (for EtO) or ISO 11137 (for radiation) and provides the necessary documentation to regulatory bodies for market clearance.
Physical Durability, Fluid Protection, and Repellency
The effectiveness of protective apparel like hospital gowns and surgical drapes depends heavily on their physical integrity and ability to repel biological fluids. A gown with a high viral filtration efficiency (VFE) but low tear strength or poor fluid resistance fails to protect the user in real-world clinical scenarios involving splashes and mechanical stress.
Therefore, medical textile testing includes a suite of physical and barrier tests tailored specifically to the functional demands of the apparel. These tests confirm that the barrier properties are maintained even when the material is subjected to typical wear and stress.
Durability and Barrier Performance Testing:
- Fluid Penetration Resistance: Tests like the AATCC 42 (water resistance: impact penetration) and AAMI PB70 (liquid barrier performance) classify the level of protection offered by hospital gowns against blood, body fluids, and pathogens. The synthetic blood penetration test (ASTM F1670/F1671) is mandatory for materials claiming blood-borne pathogen resistance.
- Tear and Tensile Strength: Measures the force required to tear or break the material. This is vital for maintaining the structural integrity of hospital gowns and drapes during use, ensuring a small tear does not lead to complete barrier failure.
- Launderability/Re-sterilization: For reusable textiles, testing must confirm that the material retains its fluid resistance, filtration performance, and dimensional stability after multiple wash and sterilization cycles. This is a complex longevity test crucial for material safety over the product’s lifecycle.
- Abrasion Resistance: Determines if the material’s barrier function is compromised after being rubbed or chafed, as would happen under a surgical table or from user movement.
By verifying these physical attributes, laboratories ensure that the barrier textiles remain effective under high-stress clinical conditions, providing reliable protection beyond basic filtration capacity.
Securing Compliance Through Specialized Medical Textile Testing
The demands placed on filters and medical textiles are unique, stringent, and directly tied to human health outcomes. A successful product line requires a dedicated and robust program for medical textile testing that verifies performance across four crucial dimensions: filtration and barrier efficacy, material safety and biocompatibility, the integrity of the sterilization process, and physical durability. From achieving a benchmark viral filtration efficiency (VFE) for surgical masks to confirming the fluid resistance of hospital gowns, the complexity of global compliance requires expert oversight. Leveraging specialized contract laboratories provides the necessary technical depth and regulatory knowledge to navigate these intricate requirements, turning compliance into a competitive advantage.
Submit a testing request today to confirm the viral filtration efficiency (VFE), validate your sterilization cycles, and guarantee the material safety of your surgical masks and hospital gowns through accredited laboratory services.
FAQ
BFE (Bacterial Filtration Efficiency) measures the material’s resistance to bacteria, which typically range from 1
to 5 μm in size. Viral Filtration Efficiency (VFE) measures resistance against much smaller viral particles, generally around 0.03 μm. Since viruses are smaller, a high VFE rating is a stronger indicator of fine particle barrier performance for surgical masks and filters.
Sterilization validation requires proving that the process achieves a specific Sterility Assurance Level (SAL), typically 10−6, under defined operating conditions. This involves testing the product’s bioburden, using highly resistant biological indicators (BIs) to challenge the cycle, and performing subsequent testing for chemical residuals (e.g., Ethylene Oxide) to confirm material safety after treatment.
The integrity of hospital gowns against fluid splashes and blood-borne pathogens is verified through liquid barrier performance tests, such as the AAMI PB70 classification. The most critical test is the synthetic blood penetration test (ASTM F1670/F1671), which determines if the material can physically block the passage of blood and other infectious liquids.
ISO 10993 is the international standard for the biological evaluation of medical devices. For textiles, it mandates a series of biocompatibility tests—such as cytotoxicity, sensitization, and irritation—to confirm that the material does not pose biological risks upon contact with the body, ensuring the textile’s overall material safety.
This article was created with the assistance of Generative AI and has undergone editorial review before publishing