Advancing Therapeutics: Liposome Research and Testing
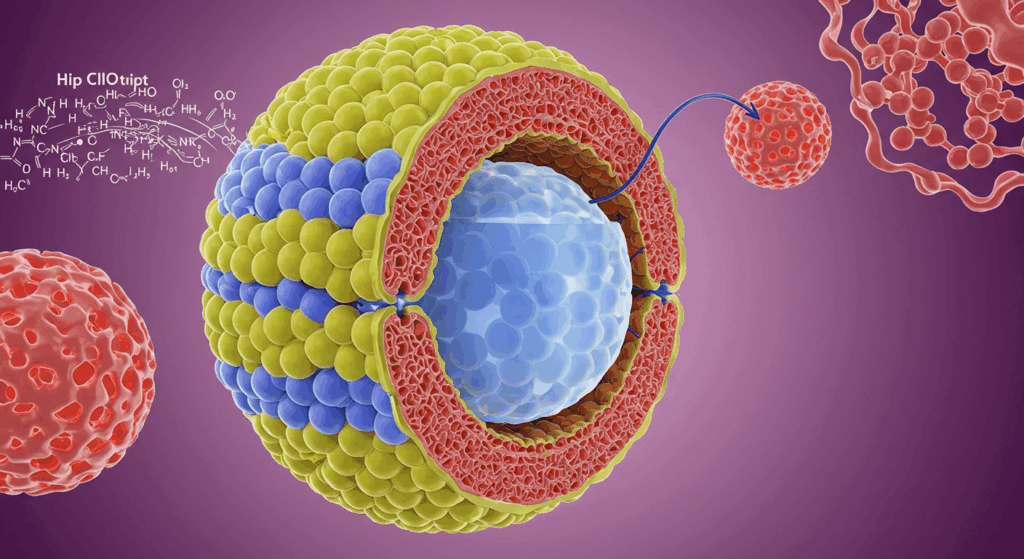
Liposomes, microscopic vesicles composed of lipid bilayers, have revolutionized the fields of medicine and biotechnology. Their unique structure allows them to encapsulate a wide range of therapeutic agents, from small molecules to large proteins and nucleic acids, protecting them from degradation and enhancing their targeted delivery. This makes liposome research a dynamic area, continuously exploring new applications and improving existing formulations. However, the successful translation of these innovative systems into clinical practice hinges on rigorous liposome testing, ensuring their efficacy, safety, and quality. This article explores the multifaceted aspects of liposome research and the critical liposome testing methodologies employed to bring these advanced drug delivery systems to fruition.
Liposome Research: Design and Formulation
The initial phase of liposome research involves the meticulous design and formulation of these vesicles. This includes selecting appropriate lipids (e.g., phospholipids, cholesterol), determining their ratios, and choosing the optimal manufacturing method (e.g., thin-film hydration, extrusion, sonication, microfluidics). The goal is to create liposomes with desired properties for specific applications, such as passive targeting to tumors or active targeting via ligands.
Key considerations in liposome design include:
- Lipid Composition: Influences membrane fluidity, stability, and interaction with biological systems.
- Size and Lamellarity: Dictated by the preparation method and affects biodistribution and cellular uptake.
- Surface Modification: Addition of polymers (e.g., PEGylation for stealth properties) or targeting ligands.
- Drug Loading: Optimizing the process to achieve high encapsulation efficiency without compromising liposome integrity.
Liposome Testing: Critical Characterization Parameters
Once formulated, comprehensive liposome testing is essential to characterize their physical and chemical properties. This characterization is vital for understanding their behavior in vitro and predicting their performance in vivo.
Crucial characterization parameters include:
- Particle Size and Size Distribution: Measured using techniques like Dynamic Light Scattering (DLS) or Nanoparticle Tracking Analysis (NTA). The particle size is critical for biodistribution, cellular uptake, and filtration.
- Zeta Potential: Determined by electrophoretic light scattering, the zeta potential indicates the surface charge of the liposomes, influencing their stability against aggregation and their interaction with biological membranes and cells.
- Encapsulation Efficiency: Quantifies the percentage of the therapeutic agent successfully entrapped within the liposomes. This is typically determined by separating encapsulated from unencapsulated drug and then quantifying the drug in both fractions.
- Drug-to-Lipid Ratio: The amount of drug loaded per unit of lipid, crucial for dosage and formulation consistency.
- Morphology: Visualized using electron microscopy (TEM, SEM) to confirm the spherical shape and lamellarity.
- Purity of Lipids: Assessed using chromatographic methods (e.g., HPLC) to ensure the quality of raw materials.
Liposome Testing: Stability and Release Studies
The stability of liposomal formulations is paramount for maintaining their integrity, preventing drug leakage, and ensuring a consistent shelf life. Furthermore, understanding the drug release studies profile is critical for predicting therapeutic efficacy.
Key aspects of stability and release studies in liposome testing:
- Physical Stability: Monitoring changes in particle size, zeta potential, and aggregation over time under various storage conditions (temperature, light, humidity).
- Chemical Stability: Assessing the degradation of lipids and encapsulated drugs using analytical techniques like HPLC, ensuring the chemical integrity of the formulation.
- Drug Retention/Leakage: Evaluating the rate at which the encapsulated drug leaks out of the liposomes under different conditions, including physiological buffers, serum, and varying temperatures.
- In Vitro Release Studies: Simulating drug release in controlled environments that mimic physiological conditions (e.g., pH, enzyme presence). This helps predict how the drug will be released once administered.
- Accelerated Stability Testing: Exposing liposomes to stressed conditions to predict long-term stability in a shorter timeframe.
Liposome Testing: Safety and Quality Control
Beyond physical and chemical attributes, liposome testing must rigorously address safety and adhere to stringent quality control measures. This ensures the product is safe for administration and consistently meets regulatory standards.
Important aspects of safety and quality control include:
- Biocompatibility: Assessing the interaction of liposomes with biological systems to ensure they do not induce adverse immune responses, cytotoxicity, or other harmful effects. In vitro assays (e.g., cell viability, hemolysis) and in vivo studies are conducted.
- Sterility Testing: For injectable or ophthalmic formulations, sterility testing is critical to confirm the absence of viable microorganisms, preventing infections. This is performed according to pharmacopoeial guidelines.
- Endotoxin Testing: Liposomes, especially those prepared from natural lipids, can be contaminated with bacterial endotoxins. Limulus Amebocyte Lysate (LAL) assay is commonly used to detect and quantify endotoxins.
- Pyrogenicity Testing: Ensuring the absence of fever-inducing substances.
- Residual Solvent Analysis: Quantifying any residual solvents from the manufacturing process to ensure they are within safe limits.
- Heavy Metal Analysis: Testing for the presence of heavy metal contaminants.
- Good Manufacturing Practices (GMP): Adherence to GMP guidelines throughout the manufacturing and liposome testing process is essential for ensuring consistent quality and safety for clinical applications.
Conclusion
The journey from liposome research to a clinically viable therapeutic product is complex, demanding a comprehensive and rigorous approach to liposome testing. From initial characterization of particle size and zeta potential to evaluating encapsulation efficiency, assessing stability, conducting detailed release studies, and ensuring biocompatibility and sterility testing, every step is critical. Adherence to strict quality control standards ensures the safety and efficacy of these advanced drug delivery systems.
If your company or research institution requires specialized liposome testing or needs to find a qualified laboratory for your liposome research projects, Contract Laboratory can assist. We connect you with a global network of accredited laboratories equipped to handle the intricate demands of liposomal analysis, helping you accelerate your advancements in drug delivery and ensure product excellence. Submit a Testing Request Today!